Prevalence and Perniciousness of Monkeypox Virus
Monkeypox is a rare disease that is caused by infection with monkeypox virus. Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. The Orthopoxvirus genus also includes variola virus, vaccinia virus and cowpox virus. Monkeypox was first discovered in 1958 when two outbreaks of a pox-like disease occurred in colonies of monkeys kept for research, hence the name ‘monkeypox.’ The first human case of monkeypox was recorded in 1970 in the Democratic Republic of the Congo. In May 2022, multiple cases of monkeypox were identified in several non-endemic countries. CDC estimated the incubation period of monkeypox virus (MPXV) using information from 22 persons with probable and confirmed monkeypox reported in the United States from May 17, 2022 – June 6, 2022.Studies are currently underway to further understand the epidemiology, sources of infection, and transmission patterns.
The continuous emergence of the MPX epidemic has attracted widespread attention around the world and has been suspected to be a potential threat to wider populations. Although smallpox vaccine has been reported to provide 85% protection against MPXV, the smallpox virus vaccination has been discontinued since 1980, when the WHO announced the eradication of smallpox virus. And there is a lack of specific drugs and vaccines to MPXV.
Genome of Monkeypox Virus
The MPXV genome is a linear, double-stranded DNA, approximately 197 kb, with inverted terminal repeats (ITRs) at its ends, and more than 190 ORFs were encoded by some MPXV strains. The non-conserved genes of the virus are generally located in ITRs at both ends, which are poxvirus species and host-specific. They are mostly associated with the immune escape of the poxvirus, such as inhibiting apoptosis, interfering with antigen presentation and recognition, and overcoming interferon (IFN) influence and disturbing with other signal paths, etc. Like all orthopoxviruses, genes encoding viral replication enzymes and structural proteins are relatively conservative, mostly located in the central region of the genome. They encode all the proteins needed for viral DNA replication, transcription, assembly, and release.
Based on genomic comparisons of several monkeypox virus strains, five genes—D10L (host range protein), D14L (complement inhibitor), B10R (apoptotic regulator), B14R (interleukin [IL]-1β binding protein), and B19R (serine protease inhibitor-like protein)—have been speculated to be most likely responsible for the increased virulence of monkeypox virus.
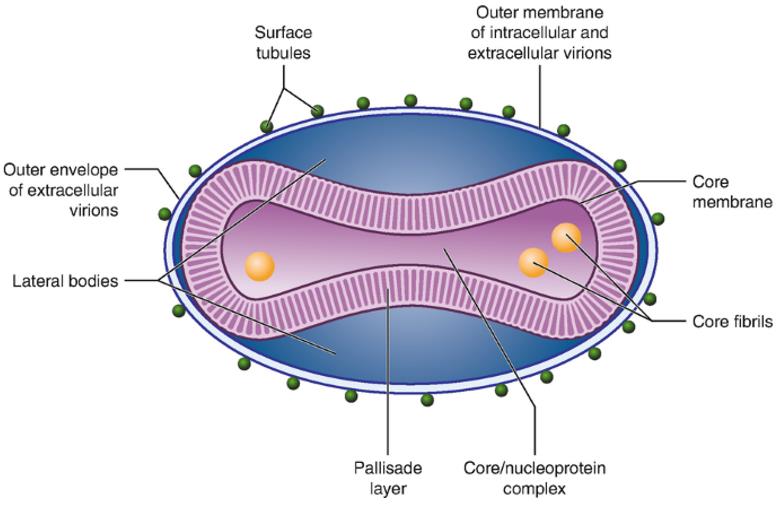
Fig.1 Structure and protein composition of MPXV
Diagnosis of Monkeypox Virus
Polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity. Cell culture provides virus strains for further characterization, but it is restricted to accredited biosafety level 3 reference laboratories. Serological testing can potentially be helpful in epidemiologic investigations, retrospective diagnosis of past infections, and diagnosis of late clinical manifestations, such as encephalitis. As orthopoxviruses are serologically cross-reactive, antigen and antibody detection methods do not provide monkeypox-specific confirmation. Serology and antigen detection methods are therefore not recommended for diagnosis or case investigation where resources are limited.
Enzyme-linked immunosorbent assay (ELISA) can be used to detect the specific IgM and IgG antibodies in the serum of MPX patients after 5- and 8-days infection, respectively. A 4-fold increase in serum antibodies at both acute and convalescent stages can be used in the diagnosis of MPXV infection. Due to the antigenic cross reaction between MPXV and other poxvirus, the specificity is insufficient. Therefore, this method cannot accurately identify MPXV and is often used in epidemiological investigation. To identify potential epitopes, candidate genes such as D2L, B18R, N2R, N3R and B21R, were identified in monkeypox that are not present in the vaccinia genome were used as overlapping peptides for screening linear antibody epitopes. Among them, B21R gene product (1,879 amino acids) was highly immunogenic, epitopes with 100% sensitivity with recent MPV has been developed and used to detect the MPV infection.
Therapeutics & Vaccine
An antiviral agent known as tecovirimat that was developed for smallpox was licensed by the European Medicines Agency (EMA) for monkeypox in 2022 based on data in animal and human studies. It is not yet widely available. Smallpox vaccine, cidofovir, ST-246, and vaccinia immune globulin (VIG) can be used to control a monkeypox outbreak. Smallpox vaccination has been reported to provide 85% protection against MPXV. Epidemiological investigations indicated that approximately 90% of confirmed MPXV cases had not been infected with other poxviruses, and most cases were born after the end of the smallpox virus eradication program, very likely having not been vaccinated with smallpox vaccine.
References
- D.B. Di Giulio, P.B. Eckburg Human monkeypox: an emerging zoonosis Lancet Infect Dis, 4 (2004), pp. 15-25.
- A. Macneil, M.G. Reynolds, Z. Braden, et al. Damon Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity Clin Infect Dis, 48 (2009), pp. e6-8
- E. Alakunle, U. Moens, G. Nchinda, M.I. Okeke Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution Viruses, 12 (2020), p. 1257
- K. Brown, P.A. Leggat Human Monkeypox: Current State of Knowledge and Implications for the Future Trop Med Infect Dis, 1 (2016), p. 8
- https://www.who.int/news-room/fact-sheets/detail/monkeypox