Introduction for anti-PEG antibodies
Polyethylene glycol (PEG), a classic non-fouling water-soluble synthetic polymer, is broadly used in the field of pharmaceutics for proteins and drug carriers1. The covalent coupling of PEG to proteins and drug carriers, a process known as PEGylation, is crucial to maintaining the colloidal stability of drugs in biological fluids and reducing their uptake by filter organs, improving their efficacy and safety after inoculation. PEG is generally considered to have non-immunogenic and to be biologically inert. However, many reports have demonstrated that PEGylated substances can elicit antibody responses against PEG (anti-PEG antibodies) compromising the therapeutic efficacy and/or the tolerance of PEGylated therapeutics2. In addition, PEG has recently been indicated as the possible cause of the anaphylactic reactions against the COVID-19 mRNA-based vaccines, in which PEG was used for coating the lipid nanoparticles (LNPs). The evidence that approximately one-third of those who experienced anaphylaxis after vaccination had a previous history of allergic or anaphylactic reactions, suggests anti-PEG antibodies formed after exposure to PEGylated compounds may play a role3. But the current evidence supporting this is contradictory. Consequently, claims that PEG is immunogenic and antigenic could lead to disappointment, particularly with an increasing number of PEGylated products such as PEGylated proteins and nanocarriers, anticipated to enter the market over the coming few years. Accordingly, a deep understanding of the incidence and clinical implications of anti-PEG immunity is a must for the continual clinical application of PEGylated therapeutics. Creative Diagnostics offers a panel of anti-PEG IgG or IgM ELISA kits with different species reactivity to detect anti-PEG IgG or IgM in Serum and plasma, including PEG antibodies detection kits. These kits can be used in scientific research and vaccine evaluation.
Background
For a long time, PEG was classified as a non-immunogenic polymer, even when administered with Freund’s adjuvant. Thanks to this inertness, it has been specifically adopted to reduce the immunogenicity of some biotech drugs. Nonetheless, in contrast to the typical assertion, a mounting of literature has emerged claiming just the opposite. Many reports have demonstrated that PEGylated substances can elicit antibody responses against PEG (anti-PEG antibodies). Such naturally occurring anti-PEG antibodies are reported to prime the host immune system against administered PEGylated therapeutics, resulting in a compromised therapeutic efficacy (Fig.1). Furthermore, Researchers have demonstrated that anti-PEG antibodies are associated with the accelerated clearance of subsequently administered doses of PEGylated nanocarriers, which is referred to as “the accelerated blood clearance” (ABC) phenomenon4.
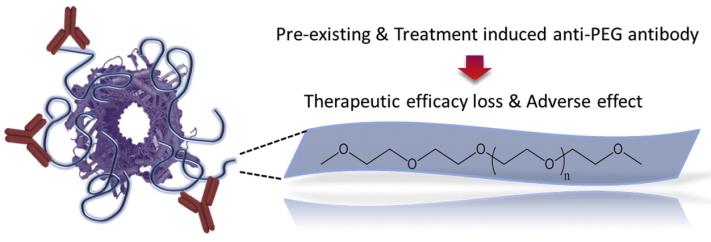
Fig. 1 The existence of anti-PEG Abs has been correlated with loss of therapeutic efficacy and increase in adverse effects5.
Anti-PEG response to PEGylated proteins
The first study demonstrating the induction of antibodies to PEG in rabbits was conducted in 19836, which was less than a decade after the introduction of protein PEGylation. In that study, free unconjugated PEG exhibited no, or very weak, immunogenicity, whereas PEG-protein conjugates induced significant anti-PEG immune responses in the immunized animals. Further studies support the scenario that the production of antibodies only occurs against PEG conjugates in a manner wherein PEG acts as a hapten. A hapten is a non-immunogenic small molecule that triggers an antibody response only when attached to an immunogenic protein that can provide CD4+ T cell epitopes, which are required to initiate the antibody response. This haptenic characteristic of PEG was further revealed by its dependence on the molecular weight and the immunogenicity of the carrier protein and/or the presence of adjuvants7.
Anti-PEG response to PEGylated nanocarriers
PEGylation is regarded as a major breakthrough in the field of nanocarrier-based therapeutics. The surface decoration of nanocarrier systems with the hydrophilic polymer PEG is crucial to maintaining the colloidal stability of nanoparticles in biological fluids and reducing their uptake by filter organs and thereby prolonging their biological half-lives. Nevertheless, despite the usefulness and importance of PEGylation, research on PEG-related immune responses has recently emphasized that the injection of PEGylated liposomes into mice can elicit a PEG-related immune response, as exemplified by the extensive production of anti-PEG IgM antibodies, which is responsible for the accelerated clearance of subsequent doses of PEGylated liposomes—the so-called “ABC” phenomenon.
PEG on anaphylactic reactions to COVID-19 mRNA vaccines
It has been widely demonstrated that PEG may enhance its immunogenic response when conjugated with other entities such as proteins, liposomes or other types of nanoparticles. More specifically, the conjugation of PEG to nanoparticles may lead to hypersensitivity reactions mainly involving the activation of the complement system, through a mechanism called complement activation-related pseudoallergy (CARPA). In the case of COVID-19 mRNA vaccines, CARPA can be activated even if the pre-existing anti-PEG antibodies have a low affinity for PEG. In fact, since multiple PEG molecules cover the LNPs surface, an early and weak anti-PEG/PEG interaction can be stabilized by the adsorption of the antibody on the lipid moiety of SLNPs, thus triggering in turn the complement activation in a subset of very sensitive individuals (Fig. 2). Accordingly, LNPs may increase the risk associated with anti-PEG antibodies compared with unbound PEGs8.
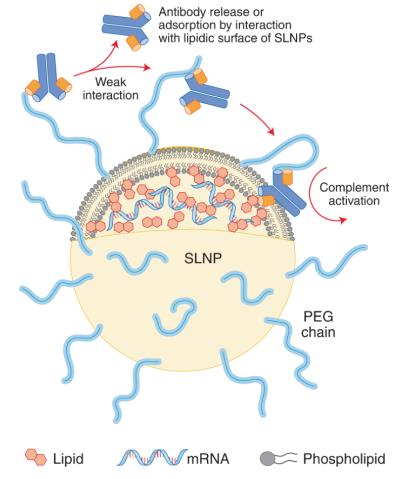
Fig. 2 Schematic representation of potential adsorption of anti-PEG antibodies on the surface of LNPs9.
A recent study correlated an episode of anaphylactic reaction after Pfizer/BioNTech vaccine in a woman with the presence of anti-PEG antibodies, determined by a skin prick test. However, the study also showed that three other subjects experiencing a similar systemic adverse reaction did not present pre-existing anti-PEG antibodies10. Overall, despite the limited number of cases described, the results of this study suggest that the role of PEG in the occurrence of anaphylactic reactions might be marginal.
Therefore, the evidence supporting PEG was the possible cause of the anaphylactic reactions against the COVID-19 mRNA-based vaccines is contradictory, and other factors might be involved. Therefore, more in-depth researches are necessary.
References
- Roberts M J, Bentley M D, Harris J M. (2002) Chemistry for peptide and protein PEGylation. Advanced drug delivery reviews, 54(4): 459-476.
- Shimizu T, Ichihara M, Yoshioka Y, et al. (2012) Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biological and Pharmaceutical Bulletin, 35(8): 1336-1342.
- Shimabukuro T T, Cole M, Su J R. (2021) Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. Jama, 325(11): 1101-1102.
- Ishida T, Maeda R, Ichihara M, et al. (2002) The accelerated clearance on repeated injection of pegylated liposomes in rats: laboratory and histopathological study. Cellular and Molecular Biology Letters, 7(2).
- Zhang P, Sun F, Liu S, et al. (2016) Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. Journal of Controlled Release, 244: 184-193.
- Richter A W, Åkerblom E. (1983) Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. International Archives of Allergy and Immunology, 70(2): 124-131.
- Ishida T, Kiwada H. (2013) Anti-polyethyleneglycol antibody response to PEGylated substances. Biological and Pharmaceutical Bulletin, 36(6): 889-891.
- Troelnikov A, Perkins G, Yuson C, et al. (2021) Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. Journal of Allergy and Clinical Immunology, 148(1): 91-95.
- Bigini P, Gobbi M, Bonati M, et al. (2021) The role and impact of polyethylene glycol on anaphylactic reactions to COVID-19 nano-vaccines. Nature Nanotechnology, 16(11): 1169-1171.
- Sellaturay P, Nasser S, Islam S, et al. (2021) Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine. Clinical and Experimental Allergy, 51(6): 861.