It was not long after the discovery of immune checkpoints (IC) that it was shown that cancer cells evolve to express more inhibitory immune checkpoint molecules to help them evade the immune system. However, ICs also give oncologists an opportunity to treat cancer in the same way: by either blocking the inhibitory molecules or by promoting the stimulation of the immune response. The groundbreaking development of IC blockade therapy with the use of monoclonal antibodies (mAbs) against PD-1, PD-L1, or CTLA-4, has revolutionized cancer treatment. In addition to the original ICs (PD-1, PD-L1, and CTLA-4), numerous other co-inhibitory (e.g. VISTA) and co-stimulatory (e.g. 4-1BB) ICs found on different cell types in the tumor microenvironment (TME) are being investigated.
Creative Diagnostics offers a number of Biosimilar Immune Checkpoint Mabs.
Background
Immune checkpoints are key regulators of the immune system. The classic immune checkpoint consists of co-stimulatory checkpoints (OX40, 4-1BB, GITR, CD40) which stimulate T cell activation and co-inhibitory checkpoints (PD-1, CTLA-4, LAG-3, TIGIT), which inhibit T-cell function.
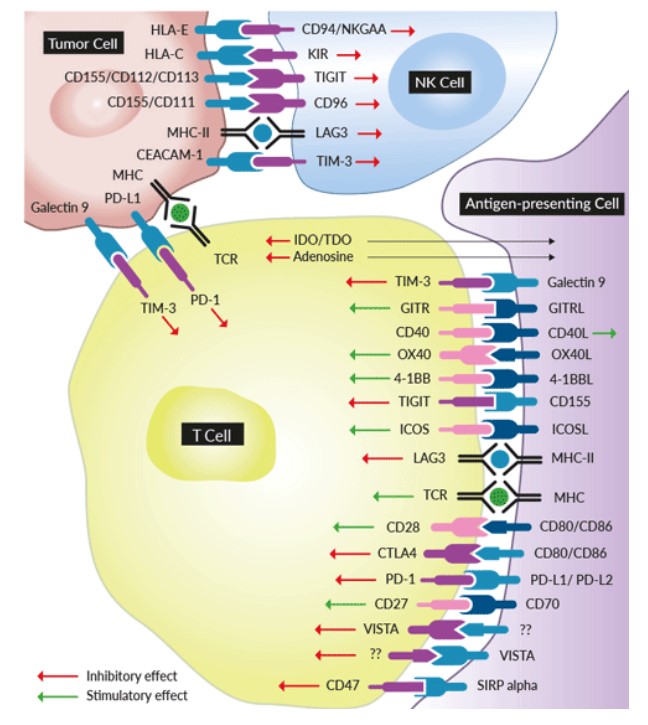
Fig 1. Multiple co-inhibitory and co-stimulatory immune checkpoint signaling
Immune checkpoints act like switches that need to be turned on (or off) to start an immune response. But cancer cells sometimes find ways to use these checkpoints to avoid being attacked by the immune system. Blocking the actions of these proteins can produce a potent anti-tumor response. This new approach to cancer treatment is referred to as cancer immunotherapy or immuno-oncology.
Medicines known as monoclonal antibodies designed to target these checkpoint proteins are called immune checkpoint inhibitors. Checkpoint inhibitors don’t kill cancer cells directly. They work by helping the immune system to better find and attack the cancer cells, wherever they are in the body. As a result, checkpoint inhibitors are able to unleash new immune responses against cancer as well as enhance existing responses to promote elimination of cancer cells.
The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011. PD-1 inhibitor pembrolizumab (Keytruda) followed shortly after, receiving approval in 2014. With more than a dozen molecules identified to be involved with signaling at the interface of T cells, the discovery of many more therapeutic leads are anticipated.
References
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015 Apr 13;27(4):450-61.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Mar 22;12(4):252-64.
- Cameron F, Whiteside G, Perry C (May 2011). Ipilimumab: first global approval. Drugs. 71 (8): 1093–104.