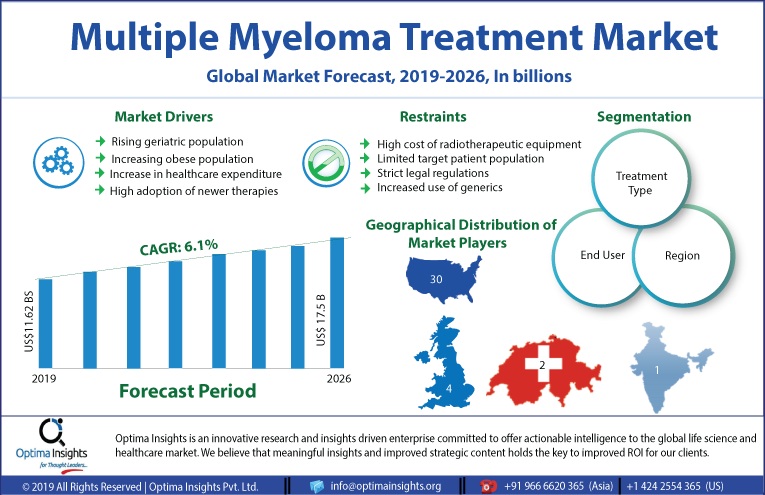
The Global Multiple Myeloma Treatment Market was valued at US$10.9 billion in the year 2018 and is likely to reach US$17.5 billion by 2026, with a CAGR of 6.1%.
Multiple Myeloma is a type of blood cancer that is characterized by cancer formation in the white blood cells otherwise denoted as the plasma cells. Generally, antibodies are produced by white blood cells for protection against foreign particles. During multiple myeloma, these plasma cells accumulate in the bone marrow outwitting the healthy cells and also produce a higher number of antibodies. These antibodies target the weaker area of the bones causing lytic lesions. Besides, these cancerous cells are also released into the bloodstream causing organ damage. Multiple Myeloma accounts for nearly 2% of the cancer-related deaths worldwide and is the second most common type of blood cancer next to Hodgkin’s Lymphoma. According to World Cancer Research Fund, in 2018, more than 159500 cases of multiple myeloma were diagnosed with the condition, where the occurrence rate among men and women was found in the ratio 1.2:1. The onset of the disease occurs after the age of 60. In recent times, the age of onset is drastically decreasing. Increasing geriatric population is one of the main factors that is set to drive this market as the aging population is set to cross 700 million by the end of 2020 and is set to cross 1.6 billion marks by 2050, according to the statistics from World Bank.
Besides, risk factors such as exposure to heavy metals, herbicides, insecticides, asbestos dust, etc. also add to the increasing burden of multiple myeloma. Also, a rise in the number of lifestyle changes, an increase in health care expenditure per capita, high adoption of new therapies and reimbursement policies are set to drive the market through the forecast period. However, the limited number of target patient population, cost of implementation of radiotherapeutic equipment, strict legal regulations, and rise in the number of patients using generic drugs are likely to dampen the market growth.
There are more than 25 FDA-approved drugs for treating multiple myeloma including therapeutics like carfilzomib, pomalidomide, panobinostat, daratumumab, ixazomib, and elotuzumab. Yet, the survival of patients with limited response or relapse while receiving treatment with primary immunodeficiency and/or immunomodulatory therapy remains poor and is one of the biggest challenges.
There are limited target patient population because the treatment of multiple myeloma has become complicated and as a consequence, results in vast heterogeneity in treatment patterns. Moreover, most of the patients diagnosed with multiple myeloma are above 65 years old. Although consensus on the timing of initiation of treatment, the choice of agents to be used, and the role of combining autologous stem cell transplant is less clear, which describes an evidence-based approach and the factors to consider upon relapse.
Recently, a combination of Bortezomib, lenalidomide, and dexamethasone (VRd) is being used to treat newly diagnosed cases of multiple myeloma and is offering promising results. According to clinicaltrials.gov more than 300 therapeutic products are spanning across more than 100 companies are in development. This highlights the investments companies have made in R&D and the interests in developing viable treatment regimens for multiple myeloma.
Monoclonal antibodies accounted for the largest market share because of its promising results in treating patients with hematological or solid malignancies by specifically targeting functional surface antigens or immune agents, leading to different mechanisms that keep the tumor at bay. It also alters the treatment landscape in cancer due to its high specificity and minimal side effect profile.
Amongst end-user, the hospital segment captured the largest market share due to rising specialist doctors providing the best chance of long-term survival. Early-stage diagnosis of multiple myeloma is one of the challenges encountered wherein it is overcome by testing for genetic mutations and molecular changes.
North American region dominates the market as the number of patients in the US is increasing YoY with approximately 14600 new cases diagnosed annually. In 2017 alone there were around 142000 patients diagnosed for multiple myeloma. This is equivalent to 40% of the total diagnosed in the 8MM (the US, Japan, France, Germany, Italy, Spain, the UK, and China). With the US being the focused market by most companies, advanced R&D infrastructure, and rise in patient population are set to drive the market.
The EU and the African population are prone to develop multiple myeloma when comparatively with the Asian countries. However, the population in the Asia Pacific region outwits the EU and the African region. Further, increasing the adoption rate of novel therapies, coupled with the support from the government as well as non-government organizations and also improving the survival of multiple myeloma patients and aging of the population.
Some of the key players of the multiple myeloma market are AbbVie, Amgen, Bristol Myers Squibb, Celgene Corporation, Cellectar Biosciences Inc., Cellular Biomedicine Group Inc, Eli Lilly and Company, Glenmark Pharma, etc.
Celgene Corporation acquired Juno Therapeutics for developing its CAR-T cell therapy portfolio as Juno Therapeutics, JCAR017 is a great fit with Celgene’s lymphoma program. Amgen acquired Nuevolution to develop small molecules drug therapeutics for chronic inflammatory disease. Eli Lilly and Company acquired ARMO Biosciences to develop immunotherapies for the treatment of cancer, fibrosis, hypercholesterolemia and inflammatory diseases.
The Multiple Myeloma Market Outlook 2019-2026, from Optima Insights, will offer a thorough insight on the market growth till the forecast year 2026 based on the market segmentation (Type, End-Use, and Region). The report also comprises comprehensive profiling of companies involved in Multiple Myeloma, product information and pipeline information.
Research Scope
- Provides detailed Analysis of the Market Structure along with forecast of the various segments and sub-segments
- Provides a Comparative Analysis of Key Marketed and Pipeline Products
- Provides Key Information on Players involved
- Provides a Complete Overview of Market Segments and the Regional Outlook
- Provides In-depth Coverage of Key News, including Major Mergers, Acquisitions and Product Development updates such as clinical trial progression updates and regulatory updates
The Report Provides Key Insights on
- History of the Multiple Myeloma Market, 2015 to 2017
- Forecast of the Multiple Myeloma Market Growth till the year 2026The key market drivers,
- restraints, challenges, future opportunities and the market dynamics driving the Multiple Myeloma Market
- Analysis of potential growth segments which will drive the market
- Landscape analysis of the major companies, and new market entrants and companies which possess disruptive technologies which can change the trend of the entire market
Key market approaches adopted by the organizations and in-depth intelligence of potential strategies which could alter the market dynamics
About Us
Optima Insights is an innovative research and insights-driven enterprise committed to offering actionable intelligence to the global life science and healthcare market. We believe that meaningful insights and improved strategic content hold the key to improved ROI for our clients. We strike an innovative engagement model with our clients to Co-Create Intelligence that would address very specific issues facing them within their functional areas. We continuously support clients through the entire journey map to enable them to make better business decisions towards attaining market leadership.
Contact
Optima Insights
Mr Chucks G
+91 966 6620 365 (Asia) | +1 424 2554 365 (US)
Email: sales@optimainsights.org
https://www.optimainsights.org