Cutaneous Mastocytosis Market 2022
The Cutaneous Mastocytosis Market is there to hit the exponential growth mark In Upcoming Years. This is the era of “online visibility”. The key stakeholders in enterprises are into an exploration of new-fangled opportunities concerning digital media, as online competition is there to rule for the next few years. With end-users turning out to be netizens, search engine optimization is of paramount importance. This would be the net-oriented trend going forward.
The global diagnosis rate as well as treatment-seeking rate of cutaneous mastocytosis is increasing rapidly, which is cited as a major factor driving the growth of the cutaneous mastocytosis treatment market over the forecast period.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/20869
- Bausch Health Companies Inc.
- Pfizer Inc.
- Merck & Co., Inc.
- Mylan N.V.
- Sanofi
- EPI Health, LLC.
- kaleo, Inc.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
- Mallinckrodt
- Bayer AG
- Johnson & Johnson
Increasing Awareness about Disease Symptoms Driving Demand Growth
Various organizations are making an extended effort to spread awareness about rare diseases such as cutaneous mastocytosis. The families of patients are trying to spread information through various social platforms, and support groups. Numerous advocacy groups are educating the general population about cutaneous mastocytosisand its treatment options.
Many controlled studies are being carried out to evaluate the actual epidemiology of the disease in different parts of the world. After the WHO updated the classification, diagnosis, and treatment approach in 2016, various countries implemented the same guidelines to improve uniformity in the diagnostic and treatment approach.
Evaluation of Novel Drug Molecules Trending among Key Companies
Several pharmaceutical manufacturers are planning to evaluate effectivity of different molecules for the treatment of cutaneous mastocytosis. A few manufacturers are already evaluating drug molecules for the treatment of cutaneous mastocytosis, and a few are seeking approvals from regulatory bodies for commencing clinical trials.
In November 2018, GT Biopharma received FDA clearance for the human phase study of First-in-Class Tri-Specific Killer Engager (TriKE), GTB-3550, for the treatment of mastocytosis. Similarly, Corbus Pharmaceuticals Holdings, Inc., a clinical phase company, is developing the drug JBT-101 for cutaneous mastocytosis treatment. That apart, various studies are being carried out by different companies for the evaluation of the effectivity of Masitinib (approved for Systemic Mastocytosis) for the treatment of cutaneous mastocytosis.
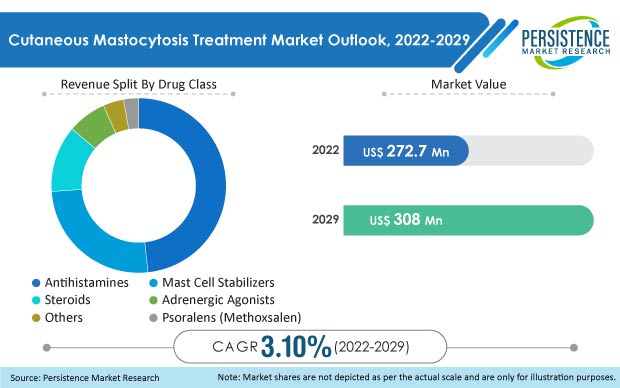
The company has segmented the global cutaneous mastocytosis treatment market based on drug class, route of administration, indication, distribution channel, and region.
- In terms of revenue, the antihistamines segment by drug class in the cutaneous mastocytosis treatment market is expected to be the dominant segment over the forecast period.
- By route of administration, the oral segment in the cutaneous mastocytosis treatment market is expected to be the most lucrative segment during the forecast period.
- By indication, the maculopapular cutaneous mastocytosis segment in the cutaneous mastocytosis treatment market is expected to hold a significant share over the forecast period.
- By distribution channel, the retail pharmacies segment is expected to be the most lucrative segment in the cutaneous mastocytosis treatment market.
- By region, the North America cutaneous mastocytosis treatment market is among the most lucrative region in the global cutaneous mastocytosis treatment market.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/20869
Augmenting R&D Investments & Favorable Reimbursement Scenario Fueling Market Growth
Increasing spending on the management of rare diseases by manufacturer as well as patients is expected to fuel the growth of the cutaneous mastocytosis treatment market. Growing research and increasing R&D funding are expected to result in increased treatment options for cutaneous mastocytosis in the coming years, driving the growth of the cutaneous mastocytosis market.
In 2016, 41.0% of new drugs approved by the FDA were orphan drugs that treat rare diseases. The seven-year market exclusivity, waive on millions of dollars in fees, as well as drug development expenses further encourages manufacturers to invest highly in rare diseases treatment market, which, in turn, is expected to drive the growth of cutaneous mastocytosis treatment market.
A favourable reimbursement scenario for rare disease treatment is further expected to enhance revenue generation in cutaneous mastocytosis treatment market. The patient pool affected by cutaneous mastocytosis is children up to the age of 14 years – over 65% to 70% of total cases.
In countries such as the United Kingdom, there is close to 100% reimbursement in most of cases for patients below 2 years of age, in cases of rare diseases such as cutaneous mastocytosis. The National Health Service (NHS) of the U.K. is undergoing substantial reforms for further improvements.
About Us:
PersistenceMarketResearch is an esteemed company with a reputation of serving clients across domains of information technology (IT), healthcare, and chemicals. Our analysts undertake painstaking primary and secondary research to provide a seamless report with a 360 degree perspective. Data is compared against rep/uted organizations, trustworthy databases, and international surveys for producing impeccable reports backed with graphical and statistical information.
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353


