Clinical Trial Management System Market 2022
New Study Reports “Clinical Trial Management System Market 2022, Global Key Players Analysis, Share, Trends, Future Opportunities Forecasts 2031″ has been Added on PMR.
Report Overview
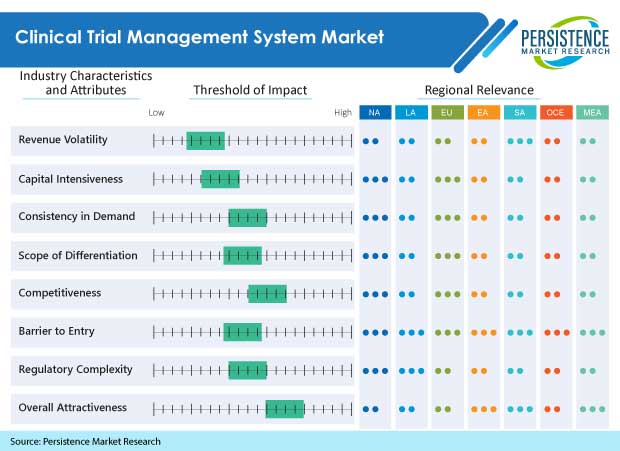
This published report for the Clinical Trial Management System Market analyzes and forecasts the marketing statistics of the product/service market on a global as well as the regional level. This detailed study Clinical Trial Management System Market also offers the previous historical data along with the forecast from 2022 to 2030. The assessment of the Clinical Trial Management System Market factors gives a brief overview of the impact on the demand over the forecast period. In addition to this, the report also studies the opportunities that are available in the report at the global level. An executive summary is also provided based on the industry snapshot for the period from 2022-2030.
Persistence Market Research has recently published a global market study on clinical trial management systems. The market is estimated to be valued at US$ 2 Bn in 2021 and is expected to surge at a CAGR of 14% from 2021 to 2031, to reach an estimated value of US$ 7 Bn by the end of 2031.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/3017
Clinical trial is a medical research study performed on humans to check the safety and efficacy of drugs, devices and therapeutic products before they are finally launched in the market. Globally, the CTMS market is witnessing significant growth due to increasing R&D investment in pharmaceutical, life science and clinical research industries.
It empowers organizations and research centers to enhance productivity and effectiveness of clinical trials by advancing and managing clinical trials.
Company Profiles:
- Oracle Corporation.
- Merge Healthcare Incorporated.
- Medidata Solutions Inc.
- PAREXEL International Corporation.
- BioClinica.
- MedNet Solutions, Inc.
- Bio-Optronics, Inc.
- DSG, Inc.
Integration of CTMS with hospital information system (HIS) provides more accurate and time saving documentation is also driving growth for the CTMS market. Additionally, increasing prevalence of diseases is supporting clinical trials in different regions, and increased clinical research outsourcing is playing a major role in the growth of the CTMS market.
Increasing regulatory requirements in many countries has resulted in increased complexity for clinical trial protocols. Presence of various end users such as pharmaceuticals, clinical research organizations (CRO) and healthcare providers has increased the acceptance of CTMS.
North America is a traditional clinical trial region. Due to regulatory and legal considerations and the clinical trial market has shifted from North America to developing countries such as India and China. Clinical trials in the U.S. have been funded and sponsored by National Institute of Health (NIH), other government agencies, academic groups, voluntary health organizations and industry.
In Europe, countries in Central and Eastern Europe provide abundant chance to life science companies for clinical development. Due to governmental support and funding for biomedical sciences, Germany has become a preferred location for clinical trials and is one of the most lucrative markets for the market.
However, Asia is the fastest growing region in the clinical trial management system market. Improved industry regulatory laws and patent expiration laws in various countries including Japan, China and India, have led to the expansion of the clinical trials market in Asia and this trend is expected to grow by the end of 2031. Asia has lower cost of conducting clinical trials compared to Europe or the U.S.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/3017
The clinical trial management system market is segmented as follows:
Delivery Mode
- Cloud based clinical trial management system
- Web based clinical trial management system
- On-premise clinical trial management system
Type
- Enterprise based clinical trial management system
- Site based clinical trial management system
End user
- Pharmaceuticals
- Clinical Research Organizations
- Healthcare providers
Component
- Software
- Hardware
- Services
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353



